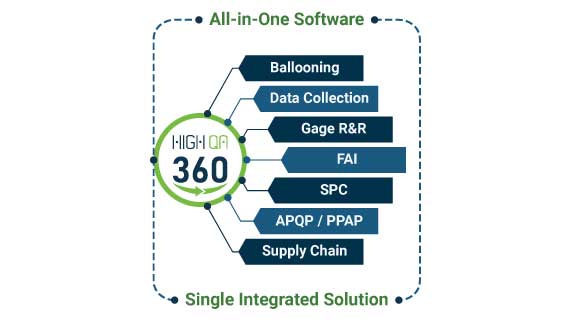
by Emily Stambaugh | Dec 5, 2024 | Webinars
WEBINAR: Transform Your Quality Process!Thursday, December 12, 2024 11:00 AM Eastern Time (US and Canada) Are you ready to revolutionize your quality process? The Transform Your Quality Process webinar recording is now available. Watch the webinar to learn how High QA...

by Emily Stambaugh | Oct 4, 2024 | Webinars
Software Transforming the Supply Chain with Manufacturing Quality at the Center!Thursday, October 3, 2024 11:00 AM Eastern Time (US and Canada) Transforming Supply Chain Transformation Webinar Manufacturing is at the heart of every product we use, yet supply chain...

by Emily Stambaugh | Jul 30, 2024 | Webinars
Achieve a 75% Faster Quality Process! – High QA Software DemoWednesday, August 7, 2024 11:00 AM Eastern Time (US and Canada) Want to speed up YOUR Quality process? Then you have come to the right place. You really can accelerate your quality process from where...

by Emily Stambaugh | Jun 18, 2024 | Webinars
Reduce Your Quality Reporting Time by 75%!Wednesday, June 26, 2024 11:00 AM Eastern Time (US and Canada) There’s a better way to manage your manufacturing quality documents. View the video below to learn how modern quality manufacturing software can save up...

by High QA | Apr 15, 2024 | Webinars
Analyze and Automate Your Data Collection Wednesday, April 24, 2024 11:00 am Eastern US View the webinar recording below to learn how modern quality manufacturing software can save up to 50% of your time spent in manufacturing quality data analysis with automation...

by High QA | Mar 13, 2024 | Webinars
5 Tips to Integrate ERP with Manufacturing Quality Tuesday, March 26, 2024 Connecting Your ERP System with Manufacturing Quality Play the free 30-minute webinar and discover 5-tips to integrate ERP with manufacturing quality. High QA’s innovative solutions digitize,...